Abstract
Background: Emicizumab, a bispecific monoclonal antibody, bridges activated factor (F)IX and FX, substituting for the function of missing activated FVIII in persons with hemophilia A (HA). The HAVEN 6 study (NCT04158648) aims to assess the safety, efficacy, pharmacokinetics (PK), and pharmacodynamics (PD) of emicizumab prophylaxis in persons with mild or moderate HA without FVIII inhibitors. Here we report the results of the interim analysis (IA).
Methods: HAVEN 6 is a Phase III, multicenter, open-label study of emicizumab in persons with mild (FVIII level >5%-<40%) or moderate (FVIII level ≥1%-≤5%) HA without FVIII inhibitors, who warrant prophylaxis as assessed by the treating physician. Participants received loading doses of emicizumab 3 mg/kg once per week (QW) for 4 weeks, followed by maintenance doses of either 1.5 mg/kg QW, 3 mg/kg every 2 weeks, or 6 mg/kg every 4 weeks. Safety endpoints include adverse events (AEs), serious AEs (SAEs), and AEs of special interest, including thromboembolic events (TEs) and thrombotic microangiopathies (TMAs). Efficacy endpoints include negative binomial-regression model estimates of annualized bleed rate (ABR) for treated bleeds, all bleeds, and joint/target joint/spontaneous bleeds; change from baseline in Hemophilia Joint Health Score 2.1 (HJHS); health-related quality of life using the Comprehensive Assessment Tool of Challenges in Hemophilia (CATCH); and treatment preference as measured by the Emicizumab Preference (EmiPref) questionnaire. PK, PD, and immunogenicity are evaluated. The IA was conducted after ≥50 participants with moderate HA completed ≥24 weeks on study or withdrew.
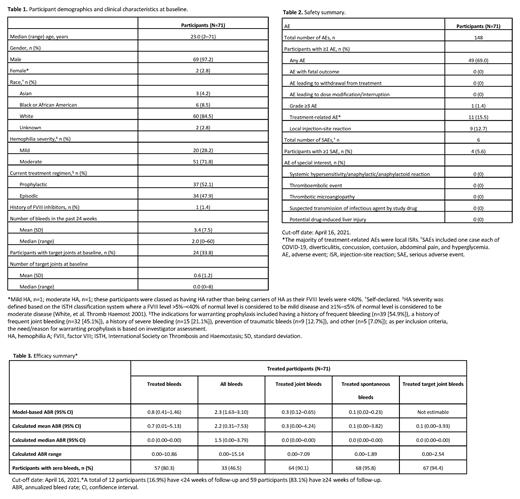
Results: Of the 72 participants enrolled, 71 received emicizumab and were included in the analysis. Median (range) follow-up period was 27.5 (6.7-61.7) weeks. Participants (69 males; 2 females) had a median (range) age of 23.0 (2-71) years (Table 1). Twenty participants (28.2%) had mild and 51 (71.8%) moderate HA; 37 participants (52.1%) were on FVIII prophylaxis at baseline. Participants had a median (range) of 2.0 (0-60) bleeds in the 24 weeks prior to emicizumab prophylaxis; 24 participants (33.8%) had target joints at baseline.
Forty-nine participants (69.0%) had ≥1 AE (Table 2); headache was the most common (14.1%). The majority of AEs (84.5%) were not emicizumab-related. Local injection-site reactions were reported for nine participants (12.7%); all were emicizumab-related. One participant (1.4%) experienced two Grade ≥3 AEs, neither emicizumab-related. Four participants (5.6%) reported a total of six SAEs; none were considered emicizumab-related by the investigator. There were no deaths, AEs leading to treatment withdrawal/modification/interruption, TEs, or TMAs.
The model-based ABR (95% confidence interval [CI]) for treated bleeds was 0.8 (0.41-1.46; Table 3). ABR (95% CI) for all bleeds was 2.3 (1.63-3.10), for treated joint bleeds 0.3 (0.12-0.65), and for treated spontaneous bleeds 0.1 (0.02-0.23). Calculated median ABRs were zero for all bleed categories except 'all bleeds'. Zero bleeds were reported for 80.3% (treated bleeds), 46.5% (all bleeds), 90.1% (treated joint bleeds), 95.8% (treated spontaneous bleeds), and 94.4% (treated target joint bleeds) of participants.
Two participants (2.8%) had anti-drug antibodies (ADAs), one of these had ADAs that were neutralizing in vitro; however, no clinical impact or impact on emicizumab PK was observed. Emicizumab trough concentrations were 10%-15% higher compared with HAVEN 1-4 (Callaghan, et al. Blood 2021).
A mean (standard deviation) improvement in HJHS total score from baseline of -1.77 (2.94) was observed at Week 25 (n=47). The trend for improvement from baseline in the treatment burden domain for CATCH was consistent across age groups (8-17 and >18 years). Except for a small improvement in 'social activity risk perception' among adolescents, the remaining CATCH domains were stable. Improvements were observed in 'treatment burden' and 'preoccupation' domains among caregivers, but due to small sample number, trends should be interpreted with caution. Overall, 48/50 (96.0%) EmiPref respondents preferred emicizumab over their previous HA therapy.
Conclusions: Data at the IA of the HAVEN 6 study indicate that emicizumab has a favorable safety profile and is efficacious in bleed prevention for persons with mild or moderate HA, which is in line with results from the HAVEN/STASEY studies.
Négrier: Biomarin: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche-Chugai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi-Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Spark: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; UniQure: Membership on an entity's Board of Directors or advisory committees. Mahlangu: Univeristy of the Witwatersrand: Current Employment; Biomarin: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Baxalta: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Catalyst Biosciences: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Research Funding; Spark: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Research Funding; Unique: Research Funding; Sanofi: Research Funding, Speakers Bureau; Takeda: Speakers Bureau; WFH: Speakers Bureau; ISTH: Speakers Bureau; Springer: Speakers Bureau. Lehle: F. Hoffmann-La Roche Ltd: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Chowdary: Bayer: Honoraria, Research Funding; CSL Behring: Honoraria, Research Funding; Freeline: Honoraria, Research Funding; Novo Nordisk: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Sobi: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Boehringer Ingelheim: Honoraria; Chugai: Honoraria; F. Hoffmann-La Roche Ltd: Honoraria; Sanofi: Honoraria; Spark: Honoraria. Catalani: F. Hoffmann-La Roche Ltd: Current Employment. Jiménez-Yuste: NovoNordisk: Consultancy, Honoraria, Research Funding; Octapharma: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Sobi: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; CSL Behring: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BioMarin: Consultancy; Sanofi: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria, Research Funding. Beckermann: F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Schmitt: F. Hoffmann-La Roche Ltd: Current Employment, Current holder of individual stocks in a privately-held company; Co-inventor: Patents & Royalties: Co-inventor of a patent relating to an anti-FIXa/FX bispecific antibody. Hermans: Cliniques Universitaires Saint-Luc: Current Employment; Bayer: Consultancy, Honoraria, Research Funding; Sobi: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria; CSL Behring: Consultancy, Honoraria; Takeda: Research Funding. Ventriglia: F. Hoffmann-La Roche Ltd: Current Employment, Other: Holds stocks. Windyga: Alfasigma: Honoraria; Aspen: Honoraria; Bayer AG: Honoraria; Octapharma: Honoraria, Research Funding; Sanofi-Aventis: Honoraria, Research Funding; Sobi: Honoraria, Research Funding; Swixx BioPharma: Honoraria; Werfen: Honoraria; Alnylam Pharmaceuticals: Research Funding; Sanofi/Genzyme: Honoraria, Research Funding; Alexion: Honoraria; Takeda: Honoraria, Research Funding; Shire: Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Honoraria, Research Funding; CSL Behring: Honoraria; Baxalta: Honoraria, Research Funding; Novo Nordisk: Honoraria, Research Funding; Rigel Pharmaceuticals: Research Funding. Kiialainen: F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. d'Oiron: Shire/Takeda: Honoraria, Research Funding; CSL Behring: Honoraria, Research Funding; LFB: Honoraria, Research Funding; Novo Nordisk: Honoraria, Research Funding; Octapharma: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Honoraria, Research Funding; Biomarin: Honoraria, Research Funding; Sobi/Sanofi: Honoraria, Research Funding; Uniqure: Honoraria; Spark: Honoraria. Moorehead: Roche Canada: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees. Teodoro: F. Hoffmann-La Roche Ltd: Current Employment. Shapiro: Sangamo: Other: Advisory board fees, Research Funding; Prometric BioTherapeutics: Research Funding; Pfizer: Research Funding; Novo Nordisk: Other: Advisory board fees, Research Funding, Speakers Bureau; Novartis: Research Funding; Kedrion Biopharma: Research Funding; Sigilon Therapeutics: Other: Advisory board fees, Research Funding; Takeda: Research Funding; OPKO: Research Funding; Octapharma: Research Funding; Glover Blood Therapeutics: Research Funding; Genentech: Other: Advisory board fees, Research Funding, Speakers Bureau; Agios: Research Funding; Daiichi Sankyo: Research Funding; Bioverativ (a Sanofi company): Other: Advisory board fees, Research Funding; BioMarin: Research Funding. Oldenburg: University Clinic Bonn AöR: Current Employment; Bayer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Biogen Idec: Consultancy, Honoraria, Speakers Bureau; Biomarin: Consultancy, Honoraria, Speakers Bureau; Biotest: Consultancy, Honoraria, Research Funding, Speakers Bureau; Chugai: Consultancy, Honoraria, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Honoraria, Research Funding, Speakers Bureau; Freeline: Consultancy, Honoraria, Speakers Bureau; Grifols: Consultancy, Honoraria, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Research Funding, Speakers Bureau; Octapharma: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Sparks: Consultancy, Honoraria, Speakers Bureau; Swedish Orphan Biovitrum: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gesellschaft für Thrombose- und Hämostaseforschung e.V.: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal